fuel-cell electric vehicle uses hydrogen fuel cells to generate electricity, which powers the electric motor. Hydrogen is stored in onboard tanks and reacts with oxygen from the air in the fuel-cell to produce electricity, emitting only water vapor as a byproduct. FCEVs are still in the early stages of commercialization and face infrastructure challenges.
How do fuel-cell electric vehicles work?
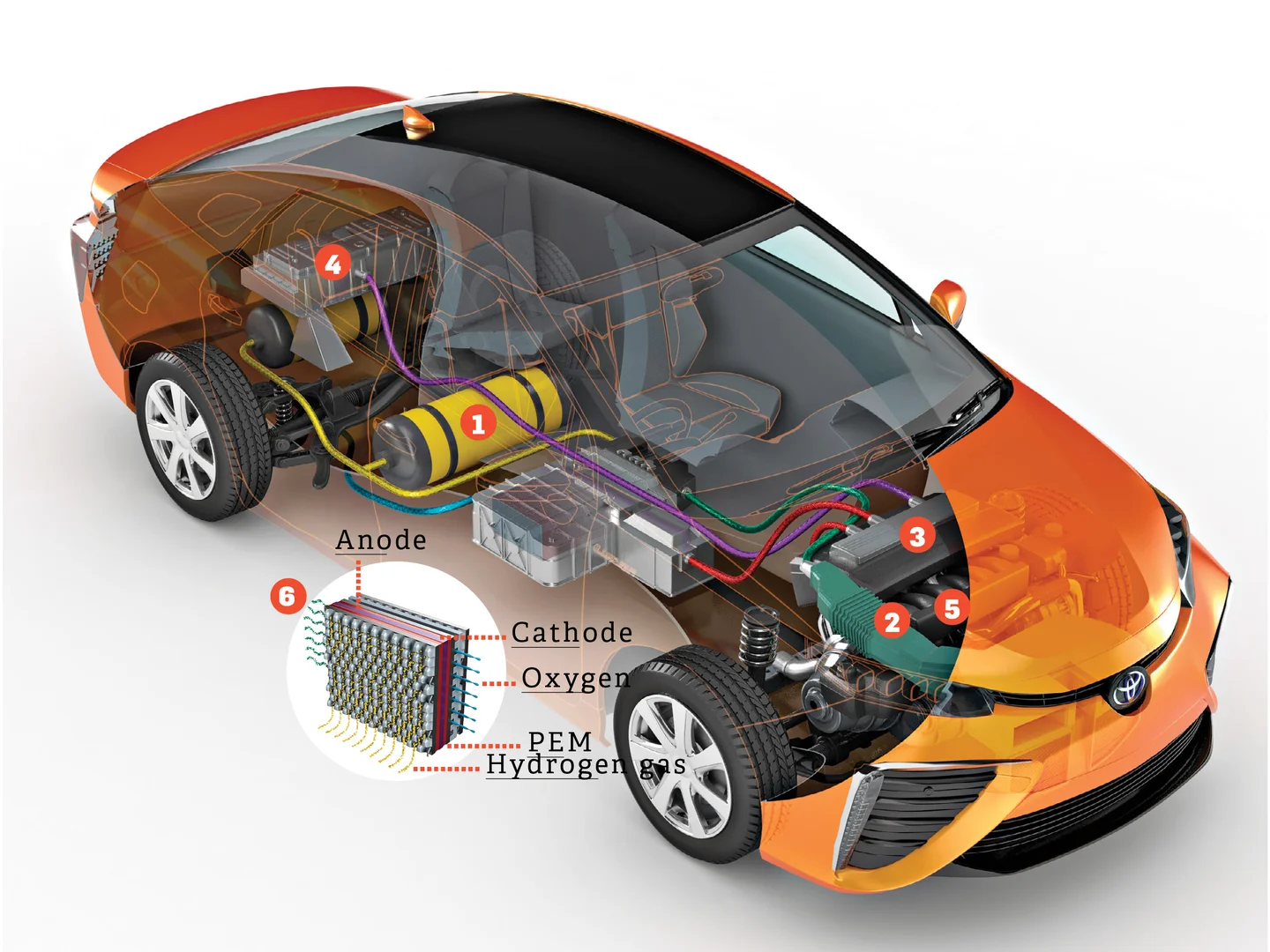
WORKING PRINCIPLE

The fuel-cell is an electrochemical device that converts the chemical energy of hydrogen into electrical energy. It consists of many individual fuel cells that are connected in series. Each fuel-cell contains an anode, a cathode, and an electrolyte membrane.
Hydrogen gas is fed into the anode, where it is split into protons and electrons. The protons pass through the electrolyte membrane to the cathode, while the electrons are forced to travel through an external circuit, generating an electric current.
At the end of the reaction the cathode, the proton, and the electrons combine with oxygen from the air to form water. As shown in the below reaction.
Cathode reaction: O2+4H+ + 4e– → 2H2O
Components of Fuel-cell electric vehicle
Hydrogen Storage
In fuel-cell electric vehicles, hydrogen is stored in high-pressure tanks or as a liquid. The hydrogen can be various methods, including natural gas reforming, electrolysis using renewable energy, or other hydrogen production processes.
The stored hydrogen is then supplied to the fuel-cell stack when the electric series comes into operation. The fuel-cell stack is a collection of individual fuel cells connected in series.
Electrochemical Reaction in the Fuel-cell Stack → Electricity Generation→ Oxygen reaction with Proton at the cathode
Inside the fuel-cell stack, a chemical reaction takes place known as electrochemical oxidation. The most common fuel-cell type for fuel-cell electric vehicles is the proton exchange membrane fuel cell.
In this type of fuel cell, hydrogen molecules (H2) are split into protons (H+) and electrons (e– ) at the anode as below.
Anode reaction: 2H2+4H+ + 4e–
The protons move through the proton exchange membrane, While the electrons are forced to travel through an external circuit, creating an electric current.
As the electrons travel through the external circuit, they generate electrical power that can be used to drive the electric motor of the vehicle.
Simultaneously, at the cathode of the fuel cell, Oxygen from the air combines with the protons that have passed through the proton exchange membrane and electrons from the electrons in the external circuit.
Cathode reaction: O2+4H+ + 4e– → 2H2O
The byproducts of the overall electrochemical reaction are water vapor and heat. This makes fuel-cell electric vehicles clean and environment-friendly technology.
Electric Motor
The electrical energy produced by the fuel-cell stack is supplied to the electric motor which drives the vehicle wheels.
The electric motor in fuel-cell electric vehicles has the same function as in battery electric vehicles smooth and efficient propulsion.
As the electric motor starts receiving electric energy from the fuel-cell vehicles start moving forward with the emission of water vapor.
Advantages of electric vehicles
- Fuel-cell electric vehicles produce zero tailpipe emissions whereas conventional internal combustion engine vehicles produce a (CO2), Nitrogen Oxide (NOX), and particulate matter.
- Reduced carbon footprints of fuel-cell electric vehicles as they depend on the source of hydrogen.
- Longer drive ranges compared to many battery-electric vehicles. This is due to the high energy density of hydrogen and the efficient electrochemical process in fuel cells.
- Fuel-cell energy efficiency compared to internal combustion engines. The electrochemical conversion of hydrogen into electricity is more efficient.
- In fuel-cell electric vehicles it takes the same time to refuel a hydrogen fuel-cell as in traditional internal combustion engines. This quick refueling time is a notable advantage compared to the longer charging time.
- It is not dependent on lithium-ion batteries. This reduces the demand for lithium, addressing the impact of lithium on the environment and disposal.
- Zero noise pollution. It is good for urban areas as there is no internal combustion. Quiet and smooth operation.
- The potential for producing hydrogen through renewable energy sources, such as electrolysis powered by solar or wind energy, improves the overall sustainability of fuel-cell electric vehicles.
Government tax benefits and subsidies to fuel-cell electric vehicles